Takeda’s enzyme replacement therapy approved by FDA for rare blood clotting disorder
Takeda’s Adzynma (ADAMTS13, recombinant-krhn) has been approved by the US Food and Drug Administration (FDA) as the first therapeutic option for congenital thrombotic thrombocytopenic purpura (cTTP), an ultra-rare inherited blood clotting disorder.
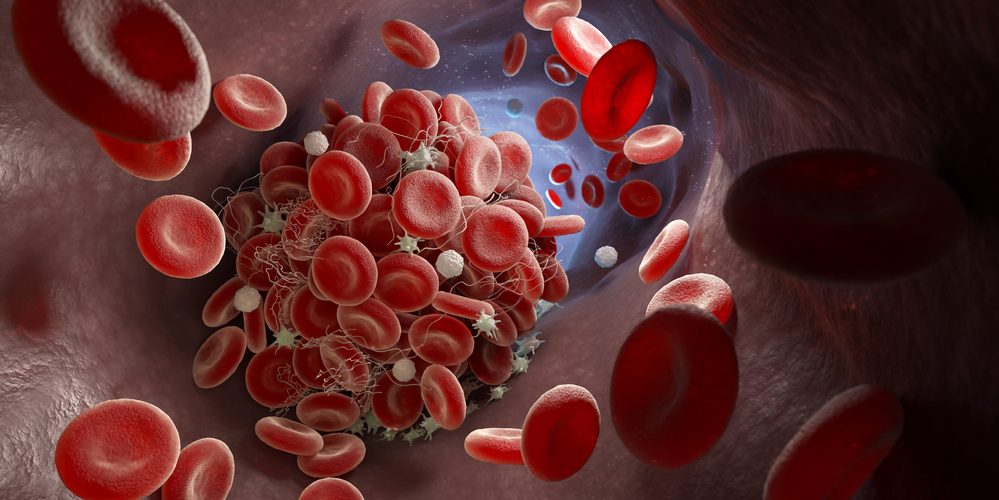
Estimated to affect fewer than 1,000 people in the US, cTTP is caused by a deficiency in the ADAMTS13 enzyme that regulates blood clotting.
Patients with cTTP can experience severe bleeding episodes, strokes and damage to vital organs, and mortality rates are high if left untreated.
“Without treatment, cTTP is ultimately fatal,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
He continued: “[The] approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
Until now, cTTP treatment has typically involved plasma therapy, which Takeda has previously described as “insufficient in restoring ADAMTS13, time-consuming and costly”.





![[onderzoek] Inzetten AI bespaart marketeers tijd](https://www.pharmamarketeer.nl/wp-content/uploads/2024/07/man-wear-casual-style-peeking-while-holding-clock-2024-07-13-02-44-40-utc-1-80x60.jpg)


