A new treatment option for adult and paediatric patients with a rare soft tissue cancer has been approved. The FDA has approved new immunotherapy drug atezolizumab (Tecentriq) for patients with advanced alveolar soft part sarcoma (ASPS) that has either spread to other parts of the body or cannot be removed by surgery for another reason.
Ook interessant voor je
Biogen ontvangt Europese marktvergunning voor Qalsody voor de behandeling van een genetische vorm van ALS
Biogen ontvangt Europese marktvergunning voor Qalsody voor de behandeling van een genetische vorm van ALS Biogen Inc. heeft aangekondigd dat de Europese Commissie (EC) een marktvergunning onder uitzonderlijke...
13 x gelezen
Terwijl tekorten toenemen, halen fabrikanten pillen van de Nederlandse markt
Terwijl tekorten toenemen, halen fabrikanten pillen van de Nederlandse markt Elk jaar halen farmaceuten honderden goedkope maar essentiële geneesmiddelen van de Nederlandse markt. Ze vinden het nutteloos om elk jaar...
20 x gelezen
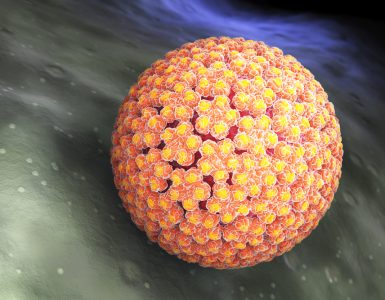
FDA Keurt Eerste Zelftestverzameling Kit voor HPV Goed
FDA Keurt Eerste Zelftestverzameling Kit voor HPV Goed De Amerikaanse Food and Drug Administration heeft een kit goedgekeurd waarmee vrouwen zelf een vaginale monster kunnen verzamelen voor HPV-screening, een stap die...
19 x gelezen






![[onderzoek] Inzetten AI bespaart marketeers tijd](https://www.pharmamarketeer.nl/wp-content/uploads/2024/07/man-wear-casual-style-peeking-while-holding-clock-2024-07-13-02-44-40-utc-1-80x60.jpg)


