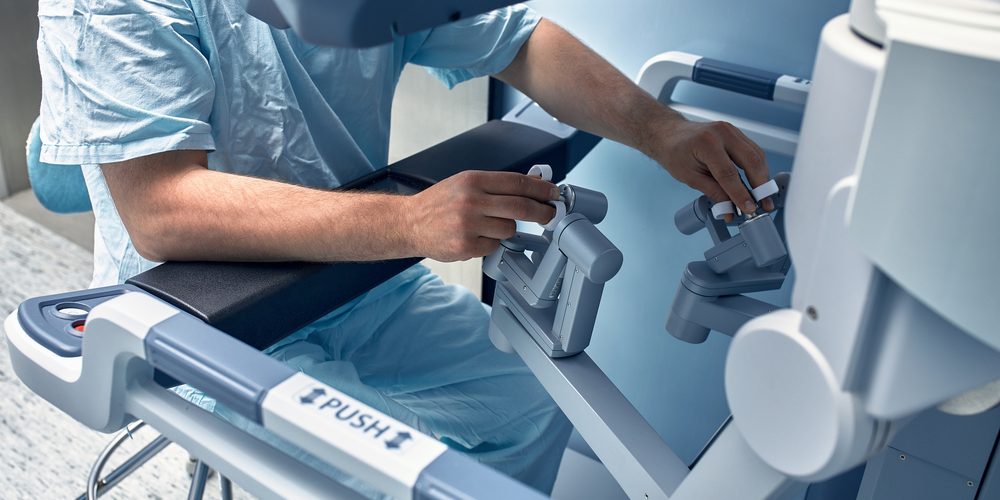
Johnson & Johnson to submit surgical robot to FDA in 2024
Johnson & Johnson MedTech have announced plans to submit the Ottava robotic surgical system to the US Food and Drug Administration (FDA) for an investigational device exemption (IDE) application in the second half of 2024.
This will allow the company to move its device into US-based clinical trials in 2024.
The Ottava robotic system integrates four robotic arms into a surgical table, able to be stowed away when not needed and easily accessible when needed. The system is intended to be highly flexible and adaptable without interrupting surgery.
If approved, the system will join Johnson & Johnson MedTech’s portfolio of robotic systems, including the Monarch Platform and the Velys Robotic-Assisted Solution.
[…]The post J&J’s to submit surgical robot to FDA in 2024 appeared first on Pharmafile.