Meningococcal ABCWY Vaccine Candidate Looks Promising in Phase 3 Trial
GSK’s investigational 5-in-1 meningococcal (MenABCWY) vaccine was found to be noninferior when compared with Bexsero (meningococcal group B vaccine) and Menveo (meningococcal group A, C, W-135, and Y conjugate vaccine), according to preliminary data presented at the 41st Annual Meeting of the European Society for Pediatric Infectious Diseases (ESPID) in Lisbon, Portugal.
The randomized, controlled, observer-blind, multi-country trial (ClinicalTrials.gov Identifier: NCT04502693) included approximately 3650 healthy individuals 10 to 25 years of age. Participants were randomly assigned to receive MenABCWY administered as 2 doses given 6 months apart or licensed meningococcal vaccines (2 doses of Bexsero plus 1 dose of Menveo).
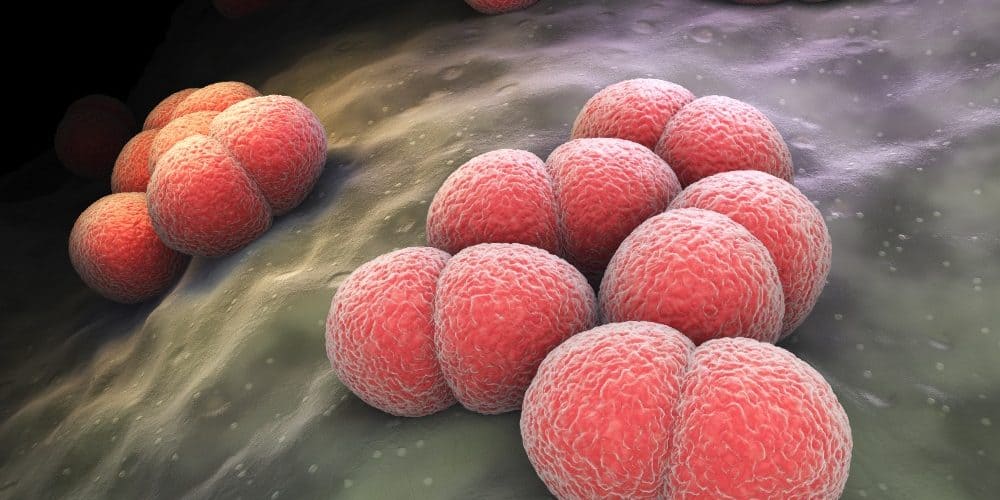
Findings showed that MenABCWY met the primary endpoint achieving noninferiority for all 5 Neisseria meningitidis serogroups (A, B, C, W and Y) compared with 2 doses of Bexsero and 1 dose of Menveo. In a separate confirmatory arm of the trial, MenABCWY demonstrated immunological effectiveness against 110 diverse meningococcal serogroup B (MenB) invasive strains. The safety profile of MenABCWY was reported to be similar to Bexsero and Menveo.
“These preliminary data further unlock the potential of our MenABCWY vaccine candidate in providing protection against invasive meningococcal disease caused by serogroups A, B, C, W and Y,” said Tony Wood, Chief Scientific Officer at GSK. “It’s particularly encouraging to see the breadth of coverage against the broadest panel of circulating MenB strains to date, as we know MenB is the most common cause of meningococcal disease in the US with the lowest immunization rate.”






![[onderzoek] Inzetten AI bespaart marketeers tijd](https://www.pharmamarketeer.nl/wp-content/uploads/2024/07/man-wear-casual-style-peeking-while-holding-clock-2024-07-13-02-44-40-utc-1-80x60.jpg)


